ATMOSPHERIC CHEMISTRY MODELLING
ATMOSPHERIC CHEMISTRY MODELLING
For many years, scientists in universities and research institutes across the world have worked on building collections of chemistry models and data for simulating atmospheric processes. These now take the form of highly complex chemical mechanisms that allow the prediction and understanding of the formation of noxious species within the atmosphere. Solving such complex chemical mechanisms requires the use of a robust and efficient mathematical integrator.
The most commonly used package for atmospheric chemistry applications is FACSIMILE for Windows. This code has been developed over several decades to focus on the solution of chemical kinetics and transport problems. It is sufficiently robust and efficient to be able to handle the extremely complex nature of atmospheric chemistry mechanisms, which can involve thousands of chemical species and tens of thousands of chemical reaction steps.
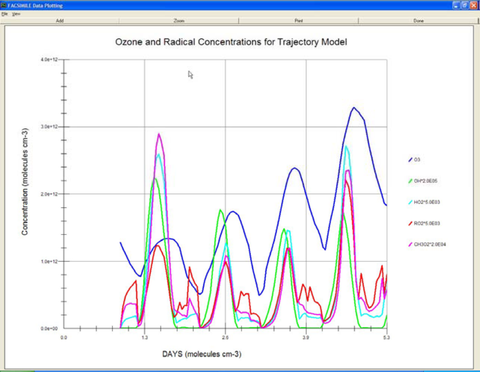
The composition of an air parcel simulated over several days using FACSIMILE
(Courtesy Mike Jenkin, Imperial College, London)
The FACSIMILE software is used by leading atmospheric chemistry modelling centres (universities and research institutes) throughout the world for studies such as:
- The formation and control of photochemical ozone on a regional scale.
- The chemistry of OH and HO2 in urban atmospheres (e.g. large cities).
- The nucleation and growth of aerosol particles.
- The formation of NOx during combustion processes.
- The formation of ozone downwind of industrial sources of hydrocarbons.
MASTER CHEMICAL MECHANISM
A complex chemical mechanism, the Master Chemical Mechanism (MCM), has been developed through a consortium involving the University of Leeds, Imperial College London, The National Environmental Technology Centre and the UK’s Meteorological Office, funded by the UK Department of Environment.
The MCM, describes the tropospheric oxidation of VOCs and is based on laboratory data and theoretical models of the component elementary reactions. Version 3 of the MCM comprises a detailed mechanism that describes the oxidation of 125 VOCs that together provide ca.91% mass coverage of the emissions of uniquely identifiable chemical species. The MCM contains in the region of 12,600 reactions and 4,500 chemical species.
The MCM is constructed to use the FACSIMILE for Windows software package for numerical integration and sensitivity analysis. The MCM, together with FACSIMILE, has been used extensively for modelling atmospheric processes. The MCM has been validated through field measurements of OH and HO2 concentrations in the atmosphere in locations such as Greece, Ireland and the UK. The measurements have been interpreted by models based on MCM and this has considerably facilitated the development of the understanding of atmospheric chemistry.
More controlled experiments have been conducted in large environmental smog chambers, such as the European Photochemical Reactor (EUPHORE) at Valencia, which provides an ideal environment for the investigation of specific organic compounds under realistic conditions, providing further validation for the MCM.
FACSIMILE for Windows and the MCM are also used in photochemical trajectory models (PTM) designed to assess the impact of individual VOCs on regional photochemical ozone production. The models operate on an idealised 5-day trajectory over large regions, and are used to calculate the increased ozone formation caused by small changes in the emissions of individual VOCs.
Calculations, using the MCM, of the potential for organic compounds to generate ozone on a regional scale have been widely used by the UK Department of Environment as aid to policy making. An alternative approach, namely integrated downwind ozone production (IDOP) from an industrial source of VOC, has also been investigated and is easier to apply in the context of regulation of industrial processes.
Further information on the MCM can be found at:
Selection of references for studies using FACSIMILE for Windows:
1. OH and HO2 chemistry in the urban atmosphere of New York City. X. Ren, H. Harder, M. Martinez, R.L.Lesher, A. Oliger, J.B. Simpas, W.H. Brune, J.J. Schwab, K.L. Demerjian, Y.Hi, X. Zhou, H. Gao. Atmospheric Environment, 37, 3639-3651, (2003).
2. Seasonal variation of VOC concentrations above a boreal coniferous forest. H Hakola, V Tarvainen, T Laurila, H Hellen, P Keronen, Atmospheric Environment, 37, 1623-1634 (2003).
3. Photochemical ozone formation in north west Europe and its control. R.G.Derwent, M.E.Jenkin, S.M.Saunders, M.J.Pilling, P.G.Simmonds, N.R.Passant, G.J.Dollard, P.Dumitrean, A.Kent. Atmospheric Environment, 37, 1983-1991, (2003).
4. Seasonal variation of peroxy radicals in the lower free troposphere based on observa tions from the FREE Tropospheric Experiments in the Swiss Alps. P. Zanis, P.S.Monks, T.J. Green, E. Schuepbach, L.J. Carpenter, G.P. Mills, A.R. Rickard and S.A. Penkett, Geo physical Research Letters. 30 (10), 1497,doi:10.1029/2003GL017122 (2003).
5. Importance of volatile organic compounds photochemistry over a forested area in central Greece. K Tsigaridis, M Kanakidou, Atmospheric Environment, 36, 3137-314(2002).
6. Development of a reduced speciated VOC degradation mechanism for use in ozone models. M.E. Jenkin, S.M. Saunders, R.G. Derwent and M.J. Pilling. Atmospheric Environment, 36, 4725-4734 (2002)
7. Comparison of measured and modeled surface ozone concentrations at two different sites in Europe during the solar eclipse on August 11, 1999. P.Zanis, C.S. Zerefos, S.Gilge, D. Melas, D. Balis, I. Ziomas, E. Gerasopoulos, P. Tzoumaka, U. Kaminski, W. Fricke. Atmospheric Environment, 35, 4663-4673, (2001).
8. Ozone formation downwind of an industrial source of hydrocarbons under European conditions, R.G.Derwent. Atmospheric Environment, 34,3689-3700, (2000).
9. Understanding radical chemistry in the marine boundary layer, Physics and Chemistry of the Earth. Carslaw N., P.J. Jacobs and M.J. Pilling Atmospheric Environmane, 25, 235-243, (2000),
10. The role of in-situ photochemistry in the control of ozone during spring at the Jung fraujoch (3,580m asl) - Comparison of model results with measurements. P. Zanis, P.S. Monks, E. Schuepbach and S.A. Penkett J.Atmos.Chem., 37(1), 1-27, (2000).
11. Modelling photochemical oxidant formation, transport, deposition and exposure of terrestrial ecosystems. D.Fowler, J.N.Cape, M.Coyle, R.I.Smith, A.-G.Hjellbrekke, D.Simpson, R.G.Derwent and C.E.Johnson. Environmental Pollution 100, 43-55, (1999).
12. Impact of aircraft emissions on tropospheric and stratospheric ozone. Part 1: Chemistry and 2-D model results. J-U Grooß, C. Brühl, T. Peter. Atmospheric Environment, 32, 3173-3184, (1998).